Defence entrance exams such as NDA entrance exam or AFCAT entrance exam or CDS entrance exam have chemistry common in their syllabus, in this article we will see some important topics that defence exam appearing aspirants must prepare also we are sharing CDS NDA AFCAT Exam Chemistry Important Formulas And Sample MCQs for year 2017.
AFCAT CDS NDA Chemistry 2017 Exam Formulas And Sample MCQs
Chemistry Important Formulas
- Measurement
The SI sytem gives the fundamental unit for each type of measurement.
Counting Significant Figures:
- If there is a decimal point anywhere in the number: Start with the first non-zero number and count all digits until the end.
- If there is not a decimal point in the number: Start with the first non-zero number and count until the last non-zero number.
Calculations with significant figures:
- Always complete calculations before rounding.
- Adding/subtracting: Answer has least number of decimal places as the problem.
- Multiplying/dividing: Answer has least number of significant figures in problem.
Tips to solve chemistry problem
Use the KUDOS method for solving word problems.
- K (Known): Use units to indentify information, Write information symbolically, Look for implied information, Write out chemical equations.
- U (Unknown): What is the problem looking for? Write information symbolically.
- D (Definition): Find equalities to convert. Choose and rearrange equations. Look for missing information in other places. If you cannot find enough information, re-evaluate your plan.
- O (Output): Plug in values to the equations, use constants as needed. Check unit cancellation and perform the calculation.
- S (Substantiation): Check validity of your answer. Check units and significant figures
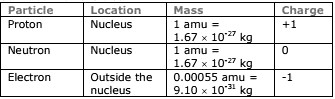
Few Facts On Atoms & Molecules
- Atoms can gain or lose electrons to form ions (atoms with a charge.
- Anion: Atom with a negative charge.
- Cation: Atom with a positive charge.
Element symbols:
- A = mass number (# of protons + # of neutrons)
- Z = atomic number (# of protons)
- C = charge (# of protons – # of electrons)
- # = number of atoms
Atoms: Made of sub-atomic particles.
Elements: Made of the same type of atom (each has the same number of protons).
Molecules: Made of more than one type of atom (more than one element) chemically bonded together.
Tips To Writing Chemical Formulas
Type 1 Binary ionic: Contains two elements—one metal and one non-metal.
1. Write the symbol and charge of the first element.
2. Write the symbol and charge of the second element.
3. Balance the charges to form a neutral compound by using subscripts.
Type 1 or 2 with Multivalent Metals
Metals that can have more than one charge.
1. The Roman numeral indicates the charge of the cation metal.
2. Follow the rules for Type #1 or Type #2 as it applies.
Type 3 Binary Covalent: Contains two non-metals (which do not form charges when bonding together).
1. Do not worry about charges with this type.
2. Write the first element’s symbol.
3. Write the second element’s symbol.
4. Use the prefixes to determine subscripts (“mono” is not used on the first element).
Acids:
1. “Acid” indicates “H+” is the cation.
2. Choose the anion:
a. “hydro__ic acid” – anion is single element (no oxygen).
b. “__ic acid” – anion is “__ate” ion
c. “__ous acid” – anion is “__ite” ion
3. Balance charges with subscripts.
The Mole
Mole: SI unit for counting (abbreviation: mol)
- 1 mole of anything = 6.02 × 1023 pieces.
- The atomic mass found on the periodic table is the mass (in grams) for 1 mole of atoms of that element.
- At standard temperature and pressure (STP), 1 mole of any gas is 22.4 L (Molar Volume of a gas)
Molar Mass (Molecular Mass, Formula Weight):
- By adding the atom masses for atoms in a molecule, the molar mass of the molecule can be found.
- Be sure to distribute subscripts outside the parenthesis to each atom inside.
Percent Composition: % = ( mass element / mass whole ) x 100
If a chemical formula is given, use atomic masses and molar mass in % composition.
Empirical formula (lowest ratio of atoms in molecule):
1. If given percent’s, assume they are grams. Change all grams to moles.
2. Divide all moles by the smallest to get the lowest ratio. Multiply by a factor if needed to make whole numbers.
3. Write the formula with the ratio as subscripts.
Molecular Formula (actual ratio of atoms in a molecule):
1. Find empirical formula, if not given to you.
2. Find the molar mass of the empirical formula.
3. Find the ratio of the molecular formula’s molar mass (given to you) to the empirical formula’s molar mass.
4. Multiple the empirical formula’s subscripts by the ratio.
Chemical Reactions
Types of reactions:
- Composition: More than one type of matter combine to form one type of matter.
- Decomposition: One type of matter decomposes into more than one type of matter.
- Single replacement: A single element changes place with an ion in a compound.
- Double replacement: Two ionic compounds switch ions.
- Neutralization reaction: Double replacement reaction with an acid and a base as the reactants.
- Redox reaction: Reduction-oxidation reaction.
- Precipitation reaction: A precipitate is formed.
Chemistry Sample Questions
Q. At constant temperature, if the pressure is doubled, the volume of the gas will be?
1. Doubled
2. Increased four times
3. Reduced by half
4. Not changed
Q. Which of the following statement Is wrong about electron?
1. It is a particle
2. It emits energy while moving in orbit.
3. Its motion is affected by magnetic field.
4. It has wave like property
Q. A strontium atom differs from a strontium ion in that the atom has a greater
1. number of electrons
2. mass number
3. number of protons
4. atomic number
Q. In Fourier’s law, the proportionality constant is called the
1. thermal conductivity
2. thermal diffusivity
3. heat transfer co-efficient
4. Stefan-Boltzman constant
Q. In a first order reaction, the concentration of the reactant decreases from 0.8 M to 0.4 M in 15 minutes. The time taken for the concentration to change from 0.1 M to 0.025 M is
1. 60 min
2. 30 min
3. 15 min
4. 7.5 min