A pall of gloom has descended over Karunapuram village in Tamil Nadu’s Kallakurichi district. As many as 24 of the 39 who have died after consuming illicit liquor are from here. A mass cremation is taking place, and many bodies are still lined up. At least one hundred people are being treated in various hospitals.
Kallakurichi Hooch Tragedy
Why In News
- A pall of gloom has descended over Karunapuram village in Tamil Nadu’s Kallakurichi district. As many as 24 of the 39 who have died after consuming illicit liquor are from here. A mass cremation is taking place, and many bodies are still lined up. At least one hundred people are being treated in various hospitals. At the Kallakurichi Government Medical College Hospital, many are battling breathlessness and loss of vision, and experiencing excruciating pain.
Steps Taken
- After the latest incident, the district superintendent of police and several police officers have been suspended and the Collector has been transferred.
- Stalin also announced the constitution of a one-man commission headed by retired justice B Gokuldoss to submit a detailed report on the incident, while Home Secretary P Amudha and Director General of Police Shankar Jiwal will submit an interim report in two days.
What Is Hooch
- Hooch is a commonly used term for poor quality alcohol, derived from Hoochinoo, a native Alaskan tribe that was known to produce very strong liquor.
- Unlike branded liquor which is produced in factories with sophisticated equipment and rigorous quality control, hooch is made in much more crude settings.
- To put it simply, hooch is alcohol meant to intoxicate. But if prepared incorrectly, it can kill. Unfortunately, it is near impossible to tell whether hooch is safe to consume without actually drinking it.
How Is Hooch Produced
- FERMENTATION: When heated, yeast reacts with sugar (from grain, fruits, sugarcane, etc.) to ferment and produce a mixture containing alcohol. This is an age-old process, used to create beverages like beer or wine. But it comes with a basic limitation. As fermentation continues, and alcohol levels rise, conditions in the mixture become toxic for the yeast. Eventually, no more fermentation can take place. Thus, to make anything stronger (above 14-18% ABC), beverages need to be distilled.
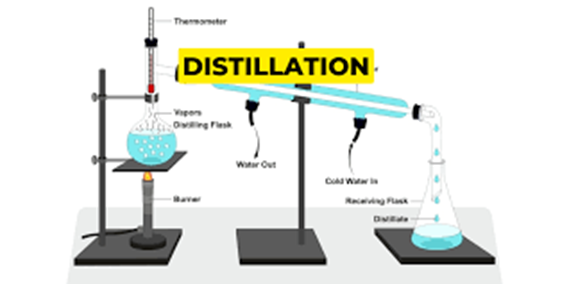
- DISTILLATION: This is the process of physically separating alcohol from a fermented mixture using evaporation and condensation. Since different parts of the mixture have different boiling points, heating it up to the correct temperature makes it possible to separate only the alcohol from the water and other remnants. Distilled beverages, or spirits, are far more potent than any fermented beverage.
- Hooch is produced using distillation of a fermented mixture, generally of locally available yeast, and sugar or fruit (often fruit waste). But the setup used for the process is very rudimentary — often just a big vat where the mixture is boiled, a pipe that captures and carries the alcoholic fumes, and another pot where concentrated alcohol condenses. Multiple rounds of distillation are carried out, to produce more potent alcohol.
Why Can Hooch Be Dangerous
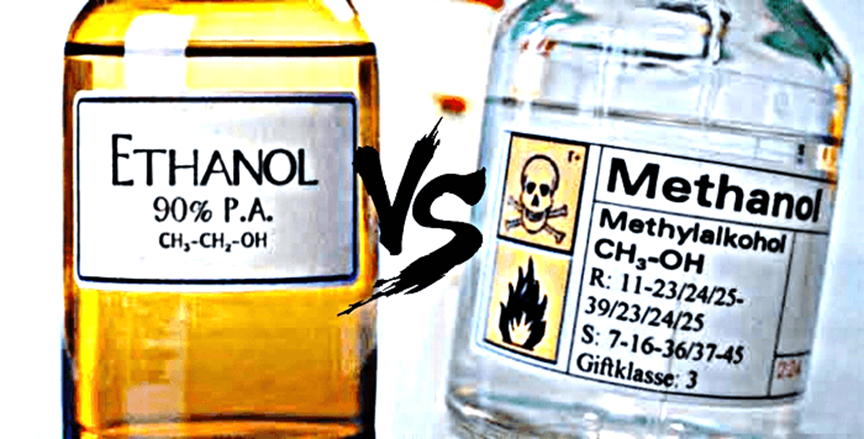
- There is an inherent risk associated with the crude methods of hooch production. The fermented mixture which is distilled contains more than just consumable alcohol (ethanol). It also contains methanol, an industrial alcohol which is highly toxic for human beings.
- Non-distilled alcoholic beverages like wine contain relatively harmless trace amounts of methanol. But during the distillation, both ethanol and methanol are concentrated. Thus, if done incorrectly, distillation can lead to an end product which contains high quantities of toxic methanol.
- Now, methanol has a boiling point of 64.7 °C, lower than that of ethanol 78.37 °C. During distillation, when the mixture reaches 64.7 °C, the pot collecting concentrated alcohol begins to fill up with a highly toxic chemical. This must be discarded for the end product to be safe.
- Further, it is crucial to maintain a temperature of above 78.37 °C but below 100°C (the boiling point of water) to obtain safe-to-consume yet potent liquor. Commercial distillers have sophisticated equipment and multiple checks to maintain the accuracy of the process.
- However, hooch-makers have no temperature control. This means that the process of distillation lacks the accuracy that is crucial to make it safe and effective.
What Other Risks Does Hooch Pose
- Beyond the risk of improperly prepared alcohol lies the risk of adulteration. Given the issues with conducting distillation without proper equipment, hooch-makers often err on the side of caution, producing an end-product that is safe, but watered down (the mixture is overboiled).
- To compensate for this, adulterants are added. Some of the known ones include organic waste, battery acid, and industry grade methanol, all of which are highly toxic.
- Adding the wrong kinds of adulterants in the wrong quantities increases the risks associated with hooch.
- First, it can make hooch far more intoxicating, producing effects such as blackouts, memory loss, and high drunkenness even on consumption of low quantities of liquor.
- Second, in extreme cases, when adulterants like methanol are present in high concentrations, the liquor is unfit to consume and can be deadly.
How Does Hooch Impact The Body
- Methanol or methyl alcohol can cause impaired vision, high toxicity and metabolic acidosis, a condition in which the body produces excessive acid that cannot be flushed out by kidneys.
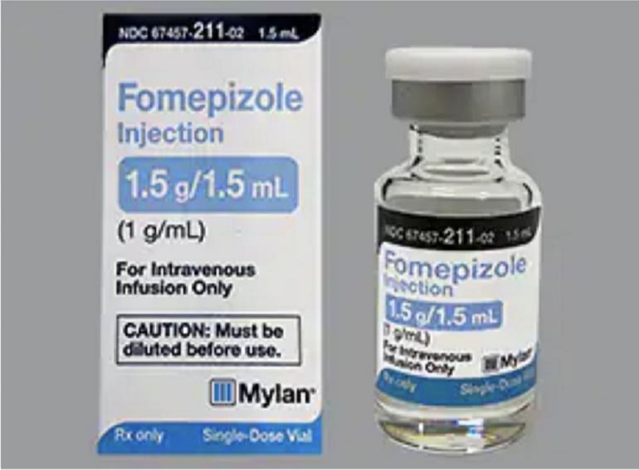
- The treatment for this is to intravenously administer Fomepizole and ethanol. However, fomepizole can be expensive and unavailable in many parts of India.
- In such cases, doctors administer a mixture of ethanol and water (1:1 ratio). Ethanol inhibits methanol’s conversion into toxins and helps in flushing it out of the body either naturally or through dialysis.