Drugs Controller General of India has approved Entod Pharmaceuticals’ PresVu eye drops, which is said to be a breakthrough in the treatment of presbyopia. The pharmaceutical company has already received approval for the eye drop from the Subject Expert Committee of the Central Drugs Standard Control Organization. PresVu is India’s first eye drop, specially made for individuals suffering from presbyopia who are forced to read with glasses.
What Is Pilocarpine ? Compound In Approved Eye Drops
Why In News
- Drugs Controller General of India has approved Entod Pharmaceuticals’ PresVu eye drops, which is said to be a breakthrough in the treatment of presbyopia. The pharmaceutical company has already received approval for the eye drop from the Subject Expert Committee of the Central Drugs Standard Control Organization. PresVu is India’s first eye drop, specially made for individuals suffering from presbyopia who are forced to read with glasses.
All You Need To Know
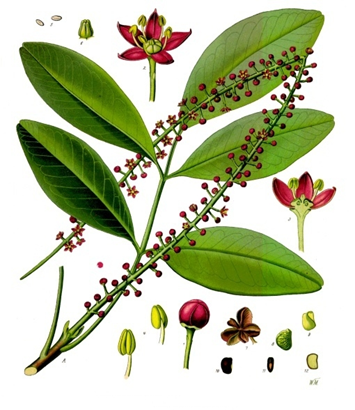
- The drug contains 1.25 percent pilocarpine hydrochloride. Pilocarpine is a plant-derived compound that has been used for decades to treat various eye conditions and dry mouth, and reduce eye pressure, among other applications.
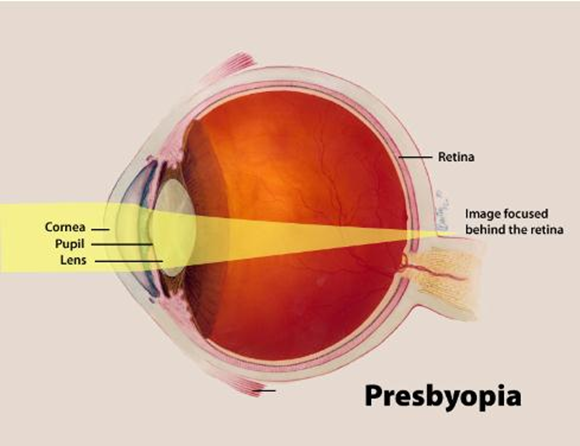
- It is meant to address presbyopia, a common age-related vision condition that typically impacts those over 40, causing the eyes to gradually lose their ability to focus on nearby objects.
- The eye drop has been developed and launched by Mumbai-based pharmaceutical firm Entod Pharmaceuticals under the brand name PresVu. A single vial of the eye drop, which is designed to last about a month, will cost Rs 345.
- A similar formulation was launched in the US in 2022 under the brand name Vuity, following regulatory approval by the US Food and Drug Administration (US FDA). Vuity is the only FDA-approved eye drop that can treat age-related blurry vision.
- It is estimated, he added, that nearly 45 percent of Indians above the age of 40 years have presbyopia. Data from phase 3 clinical trials of the drug—carried out in 250 patients across 10 sites—submitted to the DCGI showed that it works best in people aged 40 to 55 years with mild-to-intermediate presbyopia, temporarily correcting vision problems.
- The eye drop must be used only under prescription by an ophthalmologist. One drop of the drug should be put in each eye every day. The effect lasts about six hours.
- An additional drop can be put in each eye three-six hours after the first drop. This can potentially correct blurred vision for three more hours. Entod Pharmaceuticals claims that the drug starts working within 15 minutes of application but complete benefits are likely to be visible after 15 days of use.
- Regular use of pilocarpine eye drops can cause side effects such as eye irritation, increased tearing, headaches, and blurry vision in some people. “Therefore, it is not recommended for everyone,”.